knee, hip or shoulder. joint replacement implants. motion‑preserving device for the spine. (such as a spinal disc replacement) intended for contraception or prevention of sexually transmitted diseases and that. are implantable. or invasive for long-term use. active implantable medical devices. implantable.. Abstract. The present study evaluated the daily risk of healthcare-associated infections and sepsis (HAIS) events in pediatric intensive care unit patients with invasive devices. This was a retrospective cohort study. Invasive devices were associated with significant daily risk of HAIS ( p < 0.05). Endotracheal tubes posed the greatest risk of.
How to Determine the Medical Device Classification? • Follow us for more.

Surgical Tools Equipment and Medical Devices Stock Photo Image of clinic, background 168490006
Minimally Invasive Surgical Instruments Manufacturer II — SHENGWEI
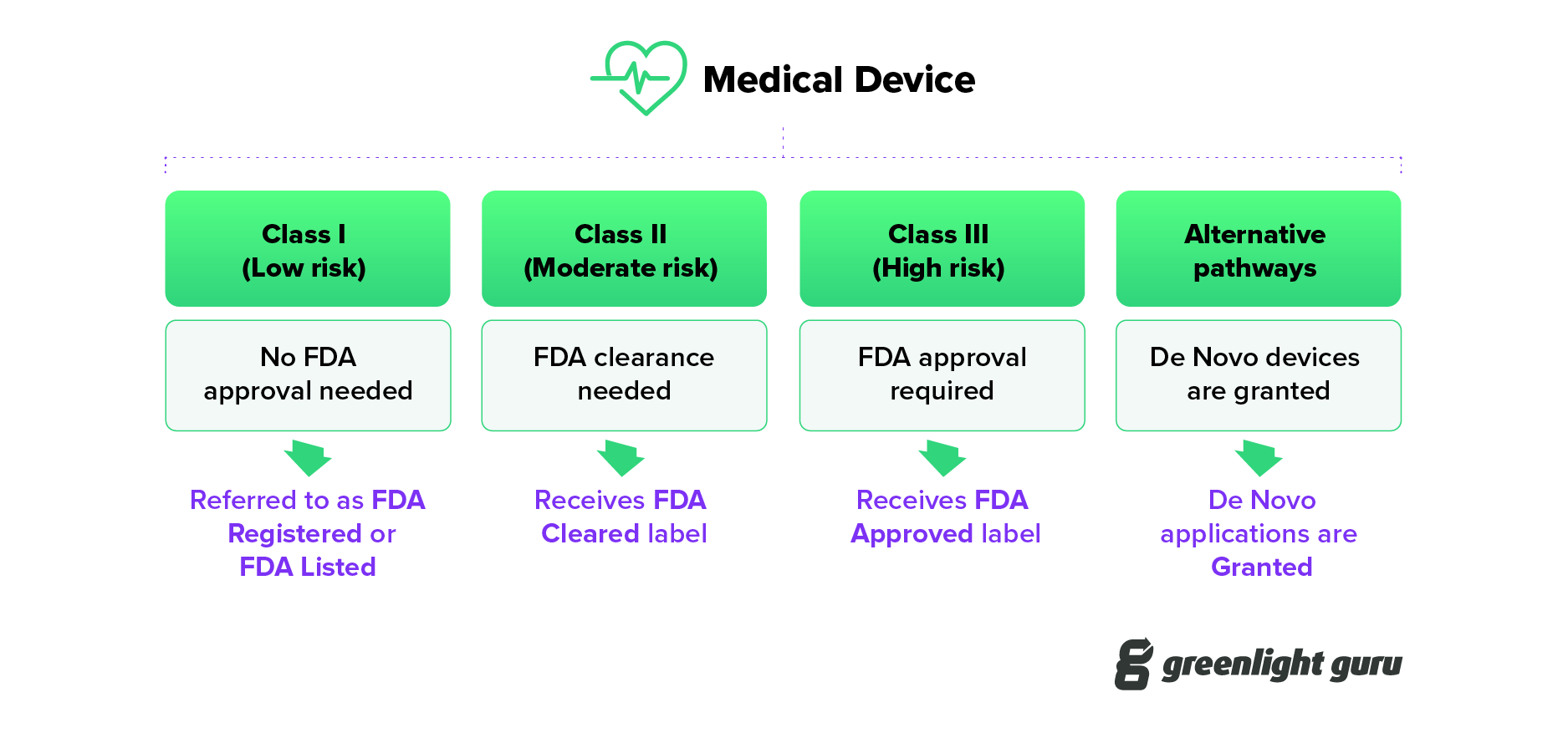
Understanding FDA Cleared vs Approved vs Granted for Medical Devices

Minimally Invasive Devices Floshield Priority Designs
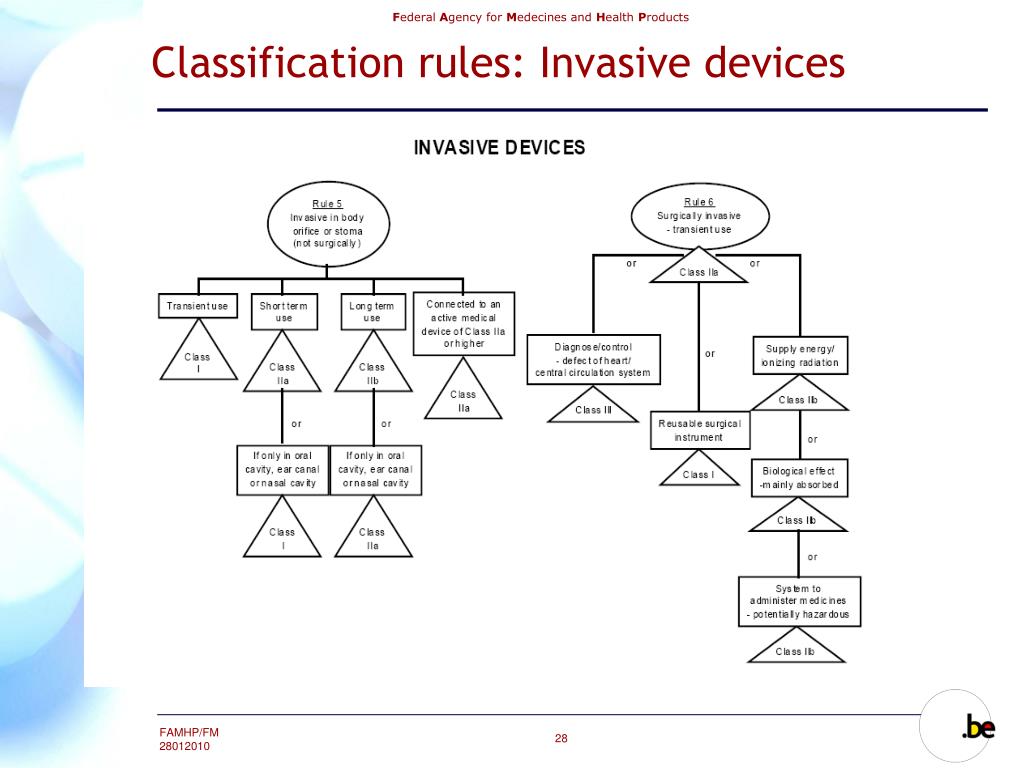
PPT Federal Agency for Medicines and Health Products PowerPoint Presentation ID6347669
FDA Guidance on Medical Device Patient Labeling Overview RegDesk

Medical Device Regulations. Design Requirements PresentationEZE
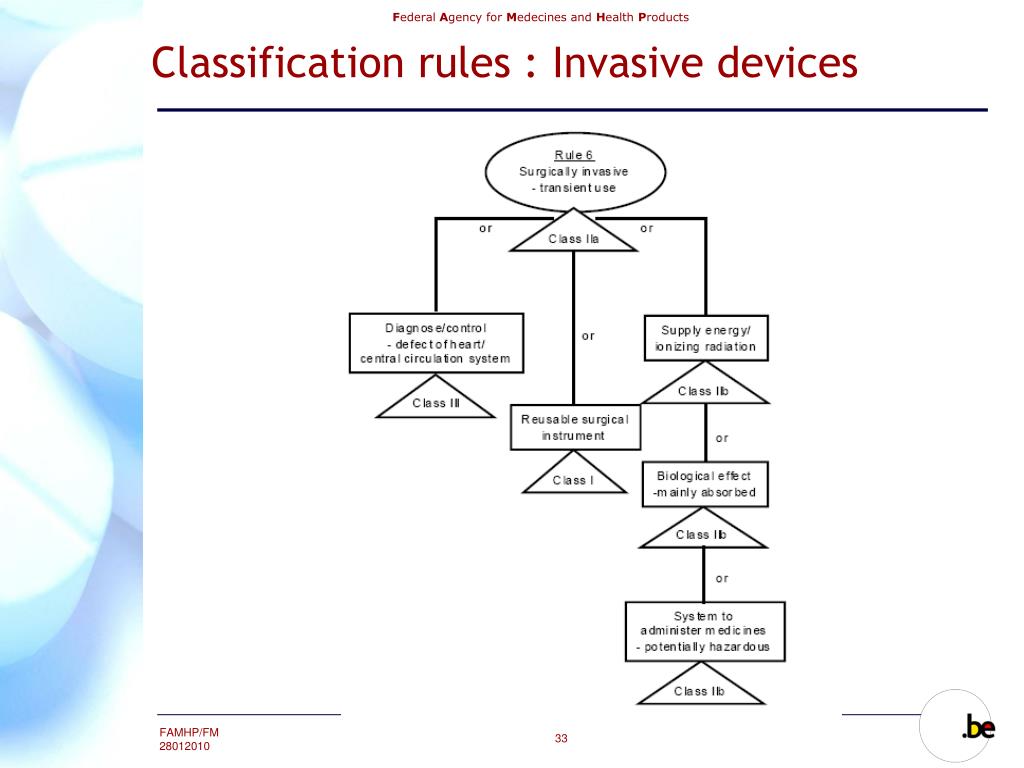
PPT Federal Agency for Medicines and Health Products PowerPoint Presentation ID6347669

Invasive devices increase risk of infection The BMJ

PPT Where the Microbes are Lurking Preventing Cross Contamination via Medical Devices

Minimally Invasive Devices Floshield Priority Designs
SAHPRA on MD/IVD classification (invasive devices) RegDesk
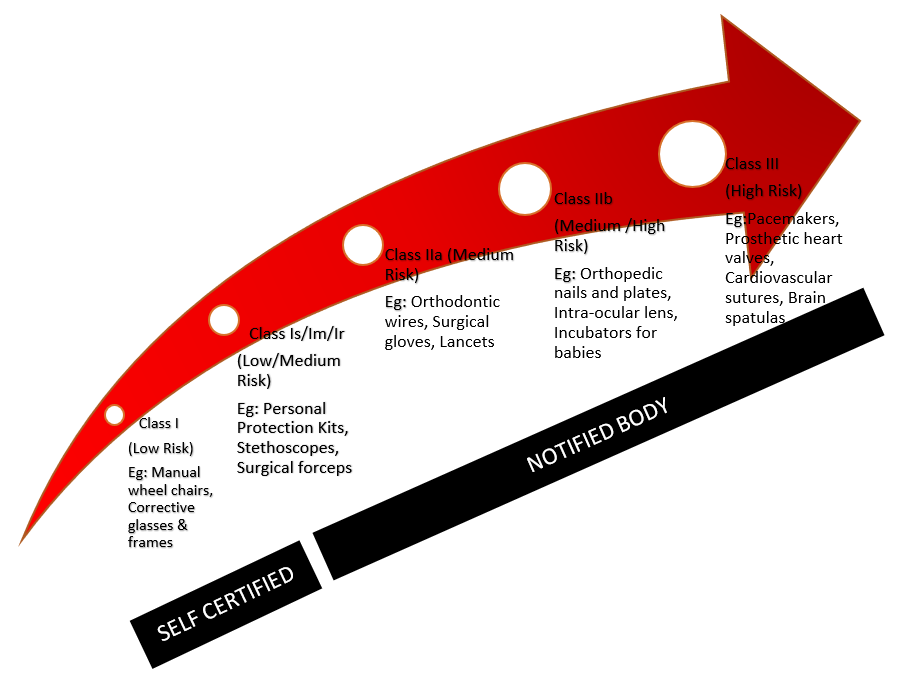
i3cglobal Medical Device Classification

Complete Guide Medical Device Classification EU MDR (Free PDF)

Checking Invasive Devices Using Chest XRays Dr. GrepMed
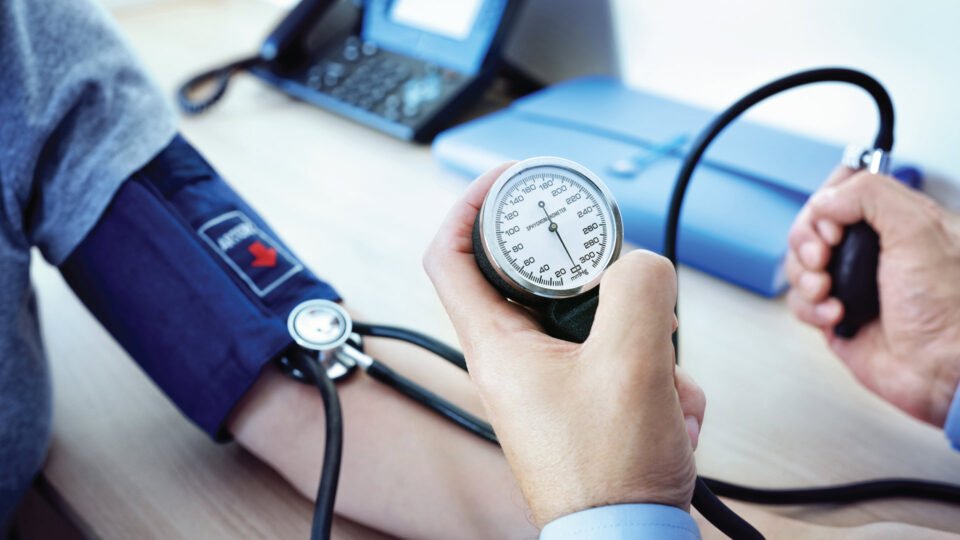
Minimally Invasive Medical Devices Reshape Patient Care Capabilities AITechPark
Invasive and NonInvasive Medical Equipment
Invasive and NonInvasive Medical Equipment
Security of Medical Devices Needs Intensive Care
2. HAIs Associated with Administration of Temporary Indwelling Devices. Several studies have identified that the kind of microorganisms causing an HAI depends on the type of medical implant inserted into the patient [17,20].Additionally, it is necessary to determine precisely whether the patient got the infection before admission to the hospital or became infected during hospitalization.. HAIs are considered an undesirable outcome, and as some are preventable, they are considered an indicator of the quality of patient care, an adverse event, and a patient safety issue.. at least 90 percent of infections were associated with invasive devices. 23 Invasive medical devices bypass the normal defense mechanism of the skin or mucous.


